Determination of Hardness in water
Objective:
To determine the amount of total hardness present in a given water sample by EDTA titration method.
Apparatus used:
Pipette, Conical flask, Burette, Beaker, Dropper etc.
Solutions used Ethyl Diamine Tetra-Acetic Acid (EDTA) solution, Water sample, Calcium carbonate solution, Ammonia buffer solution, Eriochrome Black T (EBT) as indicator Description Hardness of water is a measure of ability of water to cause precipitation of insoluble calcium and magnesium salts of higher fatty acids from soap solutions. The prescence of bicarbonates of calcium and magnesium is known as temporary hardness.The prescence of sulphates, chlorides and nitrates of calcium and magnesium is known as permanent hardness.




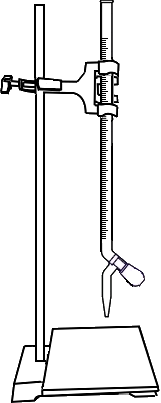
Standardisation of EDTA Solution
Fill the burette with standard EDTA solution upto zero mark.




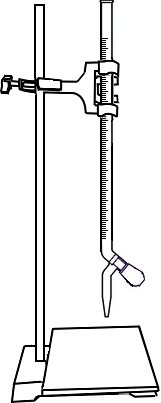




Add 10ml 0.01M Calcium Carbonate (CaCO3) solution to a conical flask through graduated pipette.



Squeeze the pipette bulb and dip pipette into the CaCO3 solution
Press the up arrow on the bulb to take the liquid up into the pipette
Add 1ml Ammonia Buffer solution by automatic pipette to the conical flask.







Ammonia buffer solution is added for
removing interference of the particle.
Add 3-4 drops of Eriochrome Black T indicator to the conical flask.



Titrate the EDTA solution till the colour changes to blue.


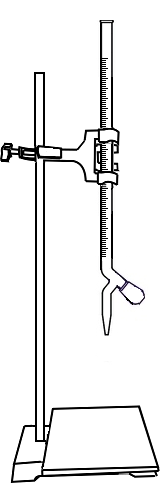




Open the knob to start the liquid running into the conical flask
Close the knob when the colour of solution in conical flask changes to pale yellow
Initial burette reading = 0 ml
Final burette reading = _______ ml
Observations:
| Volume of Calcium Carbonate (CaCo3) solution (ml) | Burette Reading (ml) | Volume of EDTA (ml) ( Final Value - Initial Value ) | |
| Initial Value | Final Value | ||

✔
✘
CaCO3 equivalent to 1ml of EDTA =
Sample Titration
Fill the burette with standard EDTA solution upto zero mark.









Add 100ml water sample to a conical flask through graduated pipette.




Squeeze the pipette bulb and dip pipette into the water sample
Press the up arrow on the bulb to take the liquid up into the pipette
Add 1ml Ammonia Buffer solution by automatic pipette to the conical flask.








Add 3-4 drops of Eriochrome Black T indicator to the conical flask.




Titrate the EDTA solution till the colour changes to blue.







Open the knob to start the liquid running into the conical flask
Close the knob when the colour of solution in conical flask changes to pale yellow
Initial burette reading = 0 ml
Initial burette reading = _______ml
Observations:
| Volume of sample taken (ml) C | Burette Reading (ml) | Volume of EDTA (ml)(Final Value-Initial Value) A | CaCO3 equivalent to 1ml of EDTA B | |
| Initial Value | Final Value | |||
✔
✘
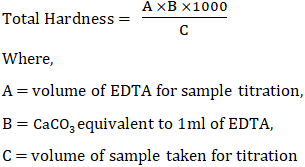
Total hardness =
Inference:
The limit of hardness as per BIS standards is 300mg/l ?
True FalseTitration =