Determination of pH
INTRODUCTION
pH of water is a measure of amount of hydrogen ions that is present in the water. It determines if the water is alkaline or acidic in nature. pH stands for potential of hydrogen. As per the World Health Organization (WHO), value of pH of drinking water should be within 6.5 to 8.5. pH can be calculated mathematically as
pH = -log [H+]
This scale was developed by the scientist Sorenson in the year 1909. The below reaction implies that the water shows that the number of H+ and OH- ions are equal in amount experimentally. It has also been proved that the product of both the concentration is equal to a constant 'K'. The value of this constant found to be between 10 and 14.
H2O –> H+ + OH-
For acids, the pH value is between 1 to 7. Alkaline solutions will have pH value from 7 to 14. There are two methods involved in the determination of pH value of water. They are
1. Colorimetric Method
2. Electrometric Method
Determination of pH is one of the important objectives in biological treatment of the wastewater. In anaerobic treatment, if the pH goes below 5 due to excess accumulation of acids, the process is severely affected. Shifting of pH beyond 5 to 10 upsets the aerobic treatment of the wastewater. In these circumstances, the pH is generally adjusted by addition of suitable acid or alkali to optimize the treatment of the wastewater. pH value or range is of immense importance for any chemical reaction. A chemical shall be highly effective at a particular pH. Chemical coagulation, disinfection, water softening and corrosion control are governed by pH adjustment. Lower value of pH below 4 will produce sour taste and higher value above 8.5 a bitter taste. Higher values of pH hasten the scale formation in water heating apparatus and also reduce the germicidal potential of chlorine.
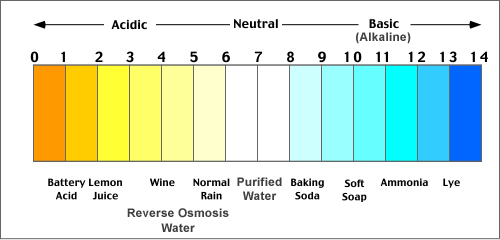
Fig : pH Scale
(Source: https://www.filterwater.com/t-ph-level-of-water.aspx)
One far end of the pH scale is not more than 1M of hydrogen ions, which results in a pH value of not more than 0. While on the other end is not more than 1M of hydroxide ions which results in a pH value of not more than 14. The pH value goes out of the 0-14 range when the concentration of the solution exceeds 1M.
Relevant Indian Standard for pH Test :
- IS 3025 (Part 11)-1983: Method of Sampling and Test (Physical and Chemical) for Water and Wastewater : pH Value (First Revision).
